Pacemaker implantation is one of the most frequently performed cardiac procedures, with 700,000+ new implants worldwide in a 2009 survey.
The benefits of implantable pacemakers are undisputed with improvements in symptoms and, for some indications, life expectancy. Traditional pacemakers consist of a battery (or generator) placed under the skin near the collarbone and connected to the heart by pacing leads. The large veins in the chest are used as conduits for the leads to access the heart (See figure 1).
Until very recently, this basic system design hadn’t changed since the inception of implantable pacemakers in the late 1950s, even though there have been many ‘hidden’ technological advancements, particularly in device software features.
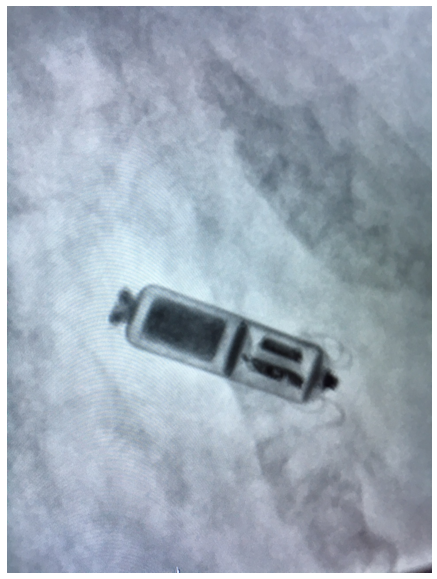
Image: A size comparison of the Medtronic Micra Leadless Pacemaker in comparison to a €1, as you can see the device is much smaller than traditional Pacemakers.


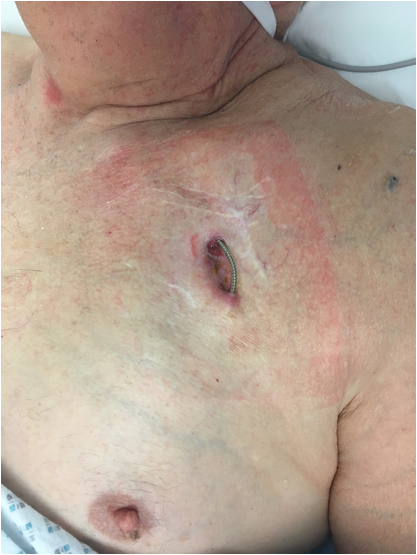
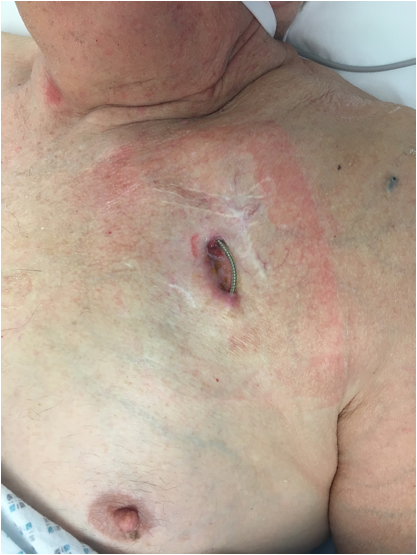
However, it has been known for some time that placement of the battery near the skin poses a significant risk for developing an infection around the pacemaker pocket (see image, left, and the table below), particularly after a pacemaker battery or lead replacement procedures.
This same flexibility is also their Achilles heel (pacing leads are often described as the ‘weak link’ of the pacemaker system) as long-term exposure to this constant movement at the shoulder and also within the heart can sometimes result in lead fracture (see figure) or insulation failure. For this reason, traditional pacing leads have a life expectancy of approximately 10 years and then require replacement and potentially extraction (which is associated with a significant risk if they have been in the body a long time).
The goal of doing away with pacing leads altogether and the generator under the skin by miniaturising the entire pacing system so it can be placed entirely within the heart has only now been realised and potentially offers a near-perfect solution to these problems.
Image: Example of a pacemaker wound infection, causing erosion of one of the pacing leads through the skin. Note the multiple scars from previous procedures crossing one another (poor surgical technique like this is a risk factor for pacemaker infection).
Comparison of possible complications relating to
Traditional Pacemaker Implants vs the Medtronic Micra:
| Traditional pacemaker | Micra | |
|---|---|---|
| Venous access: | ||
| – pneumothorax (collapsed lung) | x | |
| – Haemothorax (bleeding in chest) | x | |
| – venous obstruction | x | |
| -venous thrombosis (clot) | x | |
| – femoral vascular injury | x | |
| Lead fixation | ||
| – lead displacement | x | |
| – lead fracture | x | |
| – lead insulation failure | x | |
| -loose header connection | x | |
| – cardiac perforation/effusion | x | x |
| – Capture/sensing failure | x | x |
| – Endocarditis (heart valve infection) | x | ? |
| – valve leaflet tethering | x | |
| Pocket-related | ||
| – Infection | x | |
| - Hematoma | x | |
| – Twiddler’s Syndrome | x | |
| Device-related | ||
| – battery malfunction | x | x |
| – electrical component failure | x | x |
| – early battery depletion | x | x |
| – software malfunction | x | x |
| – mechanical integrity | x | x |
| – device emobilzation (device travels through heart/circulation) | x | |
| End of service issues | ||
| – lead extraction | x | |
| – device extraction from pocket | x |
More Information:
The Medtronic Micra Leadless pacemaker
Technological advances in electronics miniaturization and battery chemistry have now made it possible for the development of a device small enough to be implanted entirely within the heart while still providing similar battery life to a traditional pacemaker (see figure below).
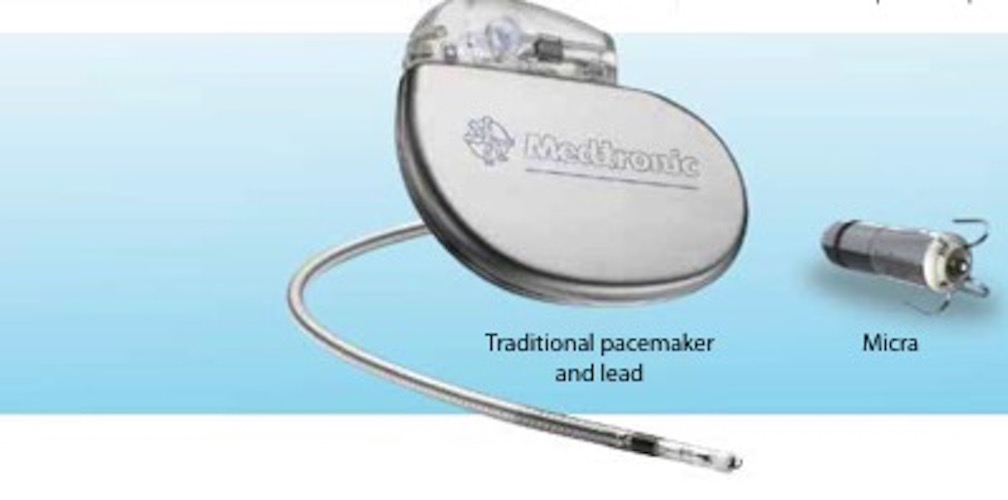
The Micra Leadless pacemaker is a miniaturised, single chamber pacemaker system – this means it is designed to pace only within the right ventricle of the heart. It is 93% smaller than traditional pacemakers.2 It is delivered via a catheter (a long plastic tube) inserted through the femoral vein at the top of the right leg and implanted directly inside the right ventricle of the heart (see figure). The Micra device eliminates the need for a separate large battery, a device pocket and insertion of a pacing lead, thereby potentially preventing many of the complications associated with traditional pacing implants but providing the same benefits.
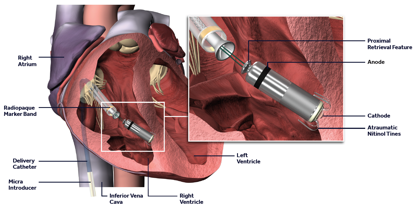
The Micra is a self-contained pacemaker, which has 4 nitinol (memory metal) wires at its tip, which help secure the device to the heart muscle and prevent it from moving subsequently. It is introduced through a large sheath inserted at the top of the right leg. The sheath has a flexible tip helping to steer the catheter to the correct position in the right ventricle (see figures). Despite the differences in size and shape, the Micra device is very similar to standard pacemakers in regards to functionality and features and, by design, is inherently MRI-conditionally safe.

Unlike traditional pacemakers, Micra can be programmed off – this is necessary when the battery expires and a new device is implanted. Animal research has demonstrated that multiple Micra devices can be implanted in the right ventricle simultaneously without compromising heart function.3
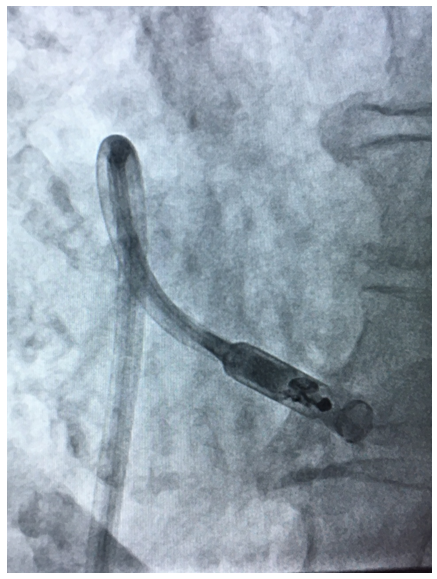
Dr Segal implanting the first Micra in a private hospital in the UK
What is the success of Micra leadless pacemaker implantation?
There was a 99.2% implant success rate in the Micra TPS pivotal study published in 2016, which recruited 726 patients worldwide and included 94 different operators.4 There were no infections or Micra dislodgements seen in the trial. In a subsequent post-approval registry of 795 patients treated by 149 different physicians in 97 countries, the implant success was 99.6%.5
What are the benefits of a Micra pacemaker
There are multiple potential benefits to Micra versus having a traditional pacemaker implant:
- No scar over the chest wall
- No pocket for the pacemaker battery (lower infection risk and no risk of bleeding or clot in the pocket requiring a repeat operation)
- Pacemaker battery not visible and battery cannot cause discomfort under the skin
- No risk of collapsed lung (pneumothorax)
- No risk of bleeding in the lung (haemothorax)
- No risk of blood vessel clot or occlusion due to the long term presence of a pacemaker lead
- No risk of pacemaker lead failure
- No risk of splinting open a tricuspid valve leaflet causing it to leak
- No need for excision of battery from pocket or lead extraction (with its associated risk)
- Longer battery life than traditional pacemakers
- Reduction in overall complication rate versus traditional pacemakers4
- Reduction in re-operation rate versus traditional pacemakers4
- Reduction in hospitalisation rate rate versus traditional pacemakers4
- Pacemaker implantation still possible even if neck and arm veins occluded from previous treatment (e.g. previous traditional pacemaker implants or long term catheter insertion, or radiotherapy)
Risks of Implantation
The overall complication rate in the Micra TPS pivotal study published in 2016 was 4.0%, which compares to a 7.6% complication rate in historical controls from traditional Medtronic pacemaker implant trials and this reflects a 48% reduction in risk.4 In a subsequent post-approval registry of 795 patients implanted with Micra treated by 149 different physicians in 97 countries, there was an overall complication rate of 1.51%, with only 1 dislodgement (0.13% frequency) and only 1 infection (0.13% frequency).5
In addition, there was an 82% reduction repeat operations with Micra versus traditional pacemakers in the Micra TPS pivotal study (0.7% vs. 3.8%, respectively) and a 47% reduction in hospitalisations (2.3% vs. 4.1%).4
Risks of Micra insertion include:
- injury to the blood vessels at the top of the right leg where the sheath is inserted (this can potentially require treatment with injection, stenting or in rare cases surgery)
- making a small hole in the heart (possibly requiring insertion of a temporary drain or in rare cases surgery)
- the development of poor electrical parameters on testing afterwards (potentially requiring device re-positioning or re-capture)
- infection in the heart (although no cases have yet occurred)
- device dislodgement
How long does the implant procedure take?
A Micra implant usually takes between 30 minutes and 1 hour and most patients are able to go home later the same day. This is identical to traditional pacemaker implants.
What is the recovery period?
Recovery is almost entirely related to recovery of the puncture site at the top of the leg and this typically needs 2 days of rest and usually a week before vigorous exercise can be recommenced.
What is the follow up for patients with Micra?
The follow up for patients implanted with Micra is identical to patients with traditional pacemakers, with a pacing check 1-month after the implant (performed wirelessly) and then subsequent checks typically every year. For information on life after pacemaker implantation, please see ‘Living with a Pacemaker’ section.
Are there alternatives to Micra?
Yes, there is an alternative leadless pacemaker called the Nanostim made by St. Jude Medical (now part of Abbott Healthcare). This is a longer device and uses a screw (or helix) at the tip to secure it to heart muscle. Other manufacturers are designing their own versions. One can also still opt for a traditional single chamber pacemaker (also known as a VVI pacemaker) (please see Pacemaker and ICD section).
Is Micra for every patient?
Micra is for patients who need a single chamber pacemaker. This includes patients with bradycardia (slow heart rhythms), where it is either unnecessary or impossible to pace the atria (the chambers at the top of the heart). Most patients who fall into this group are those who have permanent atrial fibrillation, in whom it is impossible to pace the atria when this rhythm is present. There are other reasons why a single chamber pacemaker may also be appropriate in patients without AF, particularly in the elderly, in those where mobility is very limited or for patients who require pacing very rarely.
References
- Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009–a World Society of Arrhythmia’s project. Pacing Clin Electrophysiol 2011;34:1013–27
- Williams E, Whiting J. Micratranscatheter pacing system size comparison. November 2014. Medtronic Data on File.
- Omdahl P, Eggen MD, Bonner MD, Laizzo PA, Wika K. Right ventricular anatomy can accommodate multiple Micratranscatheter pacemakers. Pacing and Clin Electrophys 2016;00:1-5
- Reynolds DW, Duray GZ, Omar R, et al. A leadless intracardiactranscatheter pacing system. N Engl J Med 2016;374:533-541
- Medtronic Global Micra Post-Approval Registry presented at Heart Rhythm Society Late-Breaking Trials May 2017, Chicago, Illinois, USA